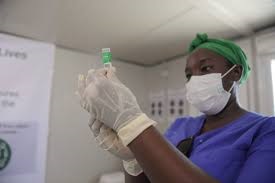
Scientists have welcomed the commencement of vaccine trials for a potential coronavirus vaccine, saying it represented a major stride towards curbing new infections and conquering the virus.
U.S. drugs maker, Pfizer and German firm, BioNTech, announced the start of the human trials on four experimental vaccines on Friday. The candidate vaccine aim to inject immune system boosters to the body, effectively fighting the virus from causing infections, Pfizer said in a statement.
The potential vaccines use the RNA technology, which is a newly developed technology meant to copy the viruses, offering better protection from diseases such as influenzas and to treat cancers.
The vaccine will enable people with no previous interaction with the virus keep themselves protected from the coronavirus.
“These are great strides in the vaccine development processes,” Mark Nanyingi, an Infectious Diseases Epidemiologist at the University of Liverpool and a lecturer at the University of Nairobi, said on Sunday.
He said while new measures such as vaccines are still being explored, efforts to curb the spread of the virus, away from pharmaceutical methods, such as social distancing, closure of schools and limiting social gatherings are critical to slow down the infections rate.
Dr John Nkengasong, the Director of the African Union’s Centre for Disease Control (Africa CDC), built with massive support from China, said the organisation is busy providing technical support to African countries to deal with the virus.
“African scientists are very capable of conducting high quality clinical trials. All trials must be scientifically sound and ethical appropriate. We expect countries to inform Africa CDC on what they are planning to do so that we can advise accordingly,” Dr Nkengasong said.
Pfizer and its German partner have launched their BNT162 trials, targeting the development of the four experimental vaccines, each of which represent different mRNA formats and target antigens.
Two of the four vaccine candidates include a nucleoside modified mRNA (modRNA), one includes a uridine containing mRNA (uRNA), and the fourth vaccine candidate utilizes self-amplifying mRNA (saRNA).
At least 25 clinical trials are already underway in Africa on a potential coronavirus vaccine, Dr Nkengasong told reporters on Thursday, during the Africa CDC weekly press briefing.
Scientists say RNA are genetic messengers which bear resemblance to the DNA.
They work by relaying the instructions of the cells triggering the production of proteins, which stops the coronavirus from causing infections.
Pfizer and BioNTech of Germany plan to distribute the vaccine worldwide. In China, Fosun Pharma, one of the world’s largest suppliers of reagents used in the testing of the virus worldwide, has entered into an agreement with BioNTech, the German firm, to sell the vaccine in the Chinese market.
The human trials for the vaccine started in the US and is expected to eventually reach 360 people. The first subjects immunized in Stage one of the study will be healthy adults 18-55 years of age.
Older adults will only be immunized with a given dose level of a vaccine candidate once testing of that candidate and dose level in younger adults has provided initial evidence of safety and immunogenicity.
For safety of the researchers and those taking part in the trials and to ensure the effectiveness of any final vaccine, only people with no exposure to the virus, determined through antibody testing, will be allowed to participate in the trial.
“There is a need to conduct large scale serological tests to determine antibody responses and general exposure of the population to the virus before relaxation of social distancing and the opening of the economy,” Dr Nanyingi wrote in an article.
In Kenya, 649 people have tested positive for coronavirus as of 9 May. The number rose to 671 on 10 May.
To curb the spread, the government continues to implement measures, including mandatory quarantines, night curfew, closure of clubs, restaurants and non-essential businesses, suspension of international flights, partial lockdowns.
Patrick Amoth, the acting Director-General of Health at the Kenyan Ministry of Health, said the World Health Organisation (WHO) has prequalified Kenya to become part of the countries where clinical trials for vaccines could take place.
The WHO approval means the relevant drugs enforcement and licensing agency in Kenya is free to provide an approval to drugs once its clinical trials show promise.
Amoth said apart from the authorisation, the health ministry has also developed clinical guidelines for healthcare workers and professionals handling the coronavirus related cases because the virus has spread within the community.
The new guidelines aim at addressing the special needs of vulnerable children, pregnant women catching the virus and other health problems arising from children and people with HIV/AIDS. Enditem